Publication of the Year 2009
S.M. Schmid, S. Kott, C. Sager, T. Huelsken, and M. Hollmann (2009).
The glutamate receptor subunit delta2 is capable of gating its intrinsic ion channel as revealed by ligand binding domain transplantation.
Proceedings of the National Academy of Sciences (USA) 106(25): 10320-10325.
doi: 10.1073/pnas.0900329106
Abstract
Press release
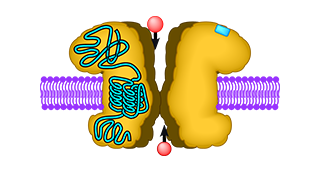
The delta2 receptor is a special member of the ionotropic glutamate receptor family. It is expressed at specific sites in the cerebellum, where it plays an important role in correct synaptogenesis during embryonal development and in the fine-tuning of motor control. Prior to our study it was unclear how delta2 served these functions, as it had been shown that delta2 did not bind any known glutamatergic agonist. That left the open question whether delta2 actually could function like a normal glutamate receptor, i.e., as a neurotransmitter-gated ion channel. To answer this question a chimaeric receptor was constructed that consisted of delta2 with a grafted glutamate-binding domain borrowed from a functional glutamate receptor. This chimaeric receptor turned out to be fully functional, an observation that conclusively proved for the first time that delta2 receptors possess a functional ion channel that only needs to be properly activated. Thus, the “problem” of the “non-functional” delta2 receptor appears to be its aberrant ligand binding site. That site may require either a very special agonist or an interaction with a modulatory protein that may act as an “on switch” for the receptor.