Publication of the Year 2007
S.M. Schmid, C. Körber, S. Herrmann, M. Werner, and M. Hollmann (2007).
A domain linking the AMPA receptor agonist binding site to the ion pore controls gating and causes lurcher properties when mutated.
Journal of Neuroscience 27(45): 12230-12241.
doi: 10.1523/jneurosci.3175-07.2007
Abstract
Press release
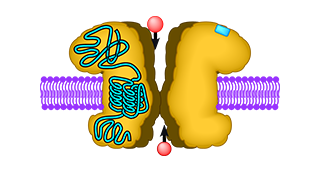
Neurotransmitter receptors at the cell surface consist of a recognition site for glutamate and a channel that opens upon glutamate binding. The glutamate recognition site and the channel are linked via an ingenious three-partite hinge mechanism, the so-called linkers A, B, and C. Linker B was known to be altered in a ceratin mouse mutant and cause a constitutively open ion channel. This blocks all normal signal transduction and causes neuronal cell death. We exchanged the three linkers A, B, and C between different types of ionotropic glutamate receptors to analyse and understand their function. Surprisingly, we found that the two least-known linkers, A and C, critically influence receptor function. Changing one of the linkers (linker A) causes a constitutively open channel as seen in the linker B mouse mutant; replacing the other linker (linker C) completely abolishes channel function. Interestingly, combining both linker mutations in one construct rescues normal ion channel function. Thus, proper interaction of the two linker regions A and C is critical for normal ion channel function. If the linkers are too stiff or sluggish, the channel will not open; if they are too loose and open too easily, a constitutively open channel might result.